
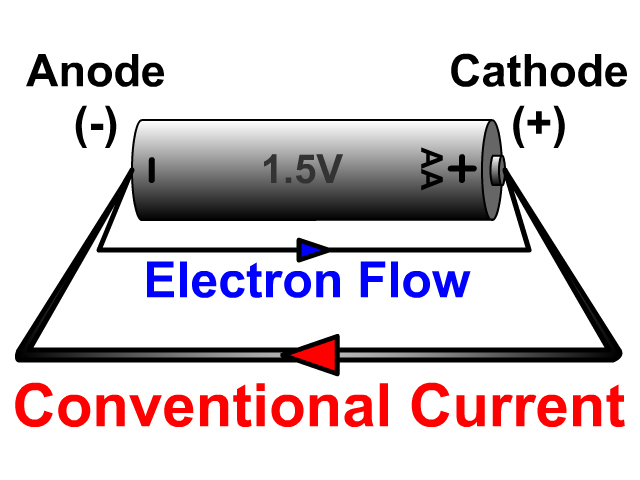
In reality, this electrode becomes an anode during charging, when the flow of current reverses, but generally, it continues to be referred to as the cathode.Ĭathode chemistry has been key to the development of lithium-ion batteries. It is, therefore, common to always refer to the cathode as the electrode which acts as a cathode during battery discharge. Although technically, a cathode is defined as an electrode where reduction reactions occur, with atoms gaining electrons, in a rechargeable battery, this process reverses. The cathode, therefore, attracts the positively charged cations.Ĭathodes are one of the key components of batteries, fuel cells, and other polarized electrical devices such as vacuum tubes. The cathode is attached to the battery’s positive terminal, but the cathode is itself polarized, with its negative end in contact with the electrolyte inside the battery. Anions, negatively charged atoms with excess electrons, are produced at the cathode and flow towards the anode.

In a battery or fuel cell, they flow through the electrolyte. Cations, positively charged atoms which have lost electrons, flow towards the cathode. Because electrons are negatively charged, electricity is conventionally considered to flow in the opposite direction to the flow of electrons.
Cathode negative or positive free#
Negatively charged free electrons flow into the positive terminal of a battery as an electrical current. A cathode is an electrode where reduction reactions occur, in which atoms gain electrons.


 0 kommentar(er)
0 kommentar(er)
